India has a problem with substandard, fake drugs. Lack of legal recall, testing makes matters worse
New Delhi: In its monthly alert for the month of August, the national drug regulator said it found 59 drugs, some of which are well-known. and sold by top pharma companies, were found to be fake or fake. Against the backdrop of the supply of drugs and cold syrups – associated with adverse events including death – to other nations, understandably, this sparked outrage.
But those fighting for patient rights and improved drug quality point out that India has bigger problems to deal with, including the lack of effective mechanisms to ensure that the country will recall such drugs.
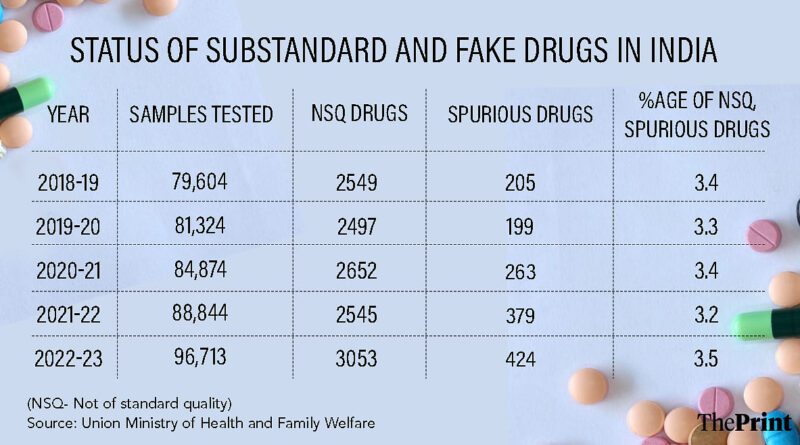
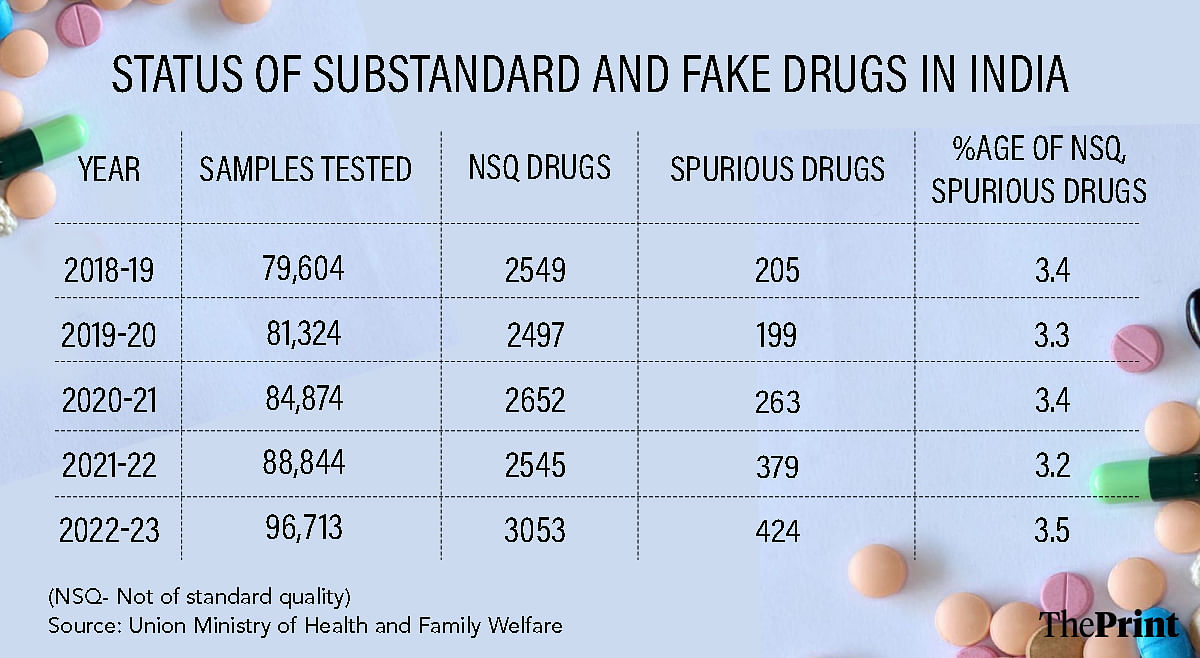
A closer look at government data showing the share of non-standard quality (NSQ) or counterfeit drugs reaching the Indian market reveals that this figure hovers between 3.2 to 3.5 percent.
Show Full Text
For example, in 2022-23, the last fiscal year for which NSQ and counterfeit drug data are available, of the 96,713 samples tested, about 3,477 or 3.5 percent were found to be problematic. In 2018-19, this number was 3.4 percent.
In the latest list issued by the Central Drugs Standard Control Organization (CDSCO) in August, 54 drugs were declared as NSQ and 5 others were fake.
NSQ drugs are those that fail to meet national or international quality standards, and therefore are ineffective or ineffective, while counterfeit drugs are those made by someone other than an authorized manufacturer, and designed to look like a real product; also, often unsafe or dangerous.
Although the list only included the names of manufacturers, not sellers, it was shown that they included products (CDSCO publishes the names of drug products found to be NSQ or fake) by pharma giants including Sun Pharma, Lupin, Torrent, Glenmark and Alkem, among others. .
For their part, many companies quickly distanced themselves from the incident saying that the groups marked are fake and not their products.
The Indian Pharmaceutical Alliance, a network of 23 top pharmaceutical distributors in the country, also issued a statement alleging falsehood and deliberate misrepresentation by select media in the face of awareness.
“The misleading proposal recklessly alters the wording of the NSQ and is wrong and affects genuine manufacturers in the production of illegal drugs,” IPA Secretary General Sudarshan Jain said in a statement issued on September 29.
Producing fake drugs is a serious crime that threatens public health, he said, adding that the connection between fake products and criminals has a strong reputation and finances. Moreover, this damages India’s reputation as a reliable supplier of medicines to the world. It is important that there is a clear distinction between NSQ and fake drugs,” the statement said.
Drugs Director General of India (DCGI) Rajeev Singh Raghuvanshi, who heads the CDSCO, said on Wednesday that the disclosure of about 50 samples found to be of low quality is part of the normal process. “Many of these drugs failed at very low levels that could pose a health risk,” he told reporters on the sidelines of the Biopharmaceutical and Life Sciences Conference organized by the Confederation of Indian Industry.
Also Read: Inside India’s shadow pharmaceutical industry – illegal drug centers, kickbacks, bad reviews
An ineffective drug recall strategy
Despite the increasing demands for laws that mandate the recall of drugs found to be ineffective or unsafe, the practices developed by CDSCO in July this year on the recall of drugs were in the form of guidelines, have no legal backing.
These trends, like the previous ones released in the last seven years, put the responsibility of remembering the producer and the advertising company.
The guidelines define two types of recall: voluntary when initiated by the licensee due to unusual observation of any quality of the product during a periodic inspection or investigation of a market complaint or defects others; and the law is regulated by drug control authorities but fails to explain how repatriation can be affected.
“We do not have a law or a law that mandates repatriation. It is a complete eyewash. I don’t understand the point of testing samples if the ones that fail are not recalled and the patients who take these drugs are not informed about their failure, “Dinesh Thakur, a public health expert focused on the development of the policy of health in America and India , said ThePrint.
Thakur, who co-authored the book The Real Pill: The Myth of Drug Control in Indiait is a complete abdication of the responsibility of the manager.
Prashant Reddy, a lawyer and co-author of the book, also stressed that the government should first recall all classes of drugs from the market and stop the licenses to manufacture them. these companies.
“The government should now conduct an analysis and publish the root cause of each failed drug, to show why exactly that drug failed the quality test. We need to know if the medicine failed because it was not made properly by the manufacturer or was stored badly while it was moving through the warehouse,” he explained.
Based on the cause of the failure and the manufacturer’s fault, the government needs to prosecute the manufacturer according to the law, he added.
Reddy even asked, what measures are being taken to inform patients who have bought medicines that these medicines are not working, adding that there is no system to deal with these issues.
But Raghuvanshi confirmed that in cases of NSQ and fake drugs, the regulator makes recommendations for prosecution or administrative action depending on how serious the non-compliance is.
When asked how the directorate ensures that doctors and the public know about drug groups published by the NSQ or false, he said the media is doing a “good job”.
“Our drug alerts are posted on our website every month and are in the public domain..even in the last 6-8 months the Press is doing a good job of reporting on these alerts,” he said. .
But Reddy insisted that the government should clarify the steps it is taking to remove the drug from the market. He said: “Just asking the companies to recall someone is useless. He added that the authorities need to ensure the return and destroy the returned groups.
What about FDC drugs?
Another major concern, said Malini Aisola, coordinator of the patient rights group All India Drug Action Network (AIDAN), is that the CDSCO’s drug warnings do not mention the names of the drug companies found. They are also less guilty of recalling drugs. relax and both manufacturers and distributors.
In India, as in many other countries, major drug manufacturers hire small companies, while managing only the marketing and distribution aspects. “We don’t know how many big companies are involved because they advertise these products, their names don’t appear on the alert list,” said Aisola.
Also, although the proportion of NSQ and fake drugs is still less than 4 percent, it is not insignificant, he added.
“Furthermore, the statistics may be misleading because the samples tested for quality largely exclude FDCs (fixed dose compounded drugs) which account for 40 percent of the drugs sold in India as there are no methods available to check their quality,” Aisola said.
FDCs contain two or more drugs in a single formulation and many of these compounded drugs available in India do not have approval from the central regulator CDSCO and are approved for manufacture by other states. and demonstrate sufficient evidence of safety and efficacy.
(Edited by Amrtansh Arora)
Also Read: Antifungals marketed as chemo, senior hospital staff involved – Delhi cancer drug cases page
#India #problem #substandard #fake #drugs #Lack #legal #recall #testing #matters #worse